
Six months after the county shut down in response the coronavirus in mid-March, Sharp Coronado CEO Susan Stone shares what the COVID-19 numbers currently look like in Coronado. 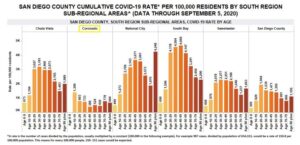 Addressing recent news coverage reporting Coronado showing an abnormally high number of cases among youth ages 10-19, Stone said that numbers for this age group have not been a concern at the hospital at any time during the pandemic. Sharp statistics, which include zip codes 92118 and 92135, show four positive cases in that age group from March through August 27, 2020. She does point out though that since Sharp Coronado doesn’t provide pediatric care, children may have received care elsewhere.
Addressing recent news coverage reporting Coronado showing an abnormally high number of cases among youth ages 10-19, Stone said that numbers for this age group have not been a concern at the hospital at any time during the pandemic. Sharp statistics, which include zip codes 92118 and 92135, show four positive cases in that age group from March through August 27, 2020. She does point out though that since Sharp Coronado doesn’t provide pediatric care, children may have received care elsewhere.
The total number of COVID-19 tests performed at Sharp Coronado Hospital as of September 14, 2020 was 10,066, with 400 positive tests (4%); and a great majority of these have not needed hospitalization. The hospital currently has one positive patient, with no other possible COVID patients awaiting results. Since the start of the pandemic, the hospital has had three COVID-related deaths.
The nasopharyngeal tests are invasive and uncomfortable for most patients, but testing technology has improved and now allows the more comfortable nasal (anterial nares) COVID testing method. This can be done by a healthcare professional, or self-administered but witnessed by a healthcare professional. Sharp recommends that individuals seeking COVID testing make sure that the lab involved is a CLIA-licensed laboratory. Sharp continues to expand testing capability as there is still a need to provide more tests. “The needed equipment is on order and expected to arrive in November,” says Stone, who points to delays related to testing supplies and equipment availability as well as Federal Emergency Management Agency (FEMA) allocations being stretched due to high demand.
Stone credits the Sharp supply chain services for doing an outstanding job adhering to the regulation changes and getting needed PPE equipment. Keeping up on the ever-changing regulatory guidelines to keep staff and patients safe is a top priority.
When asked who should get the antibody test, Stone explains that the Sharp Infection Prevention Committee has extensively evaluated this and has come up with two recommended scenarios. The first is to rule out multi system inflammatory syndrome in children (MIS-C). The second is for inpatients who are exhibiting symptoms of COVID, but whose COVID tests have repeatedly come back negative. An antibody test may indicate potential COVID exposure, however is not helpful for clinical diagnostic purposes and is therefore not used in the hospital setting. Both the CDC and FDA do not endorse antibody testing for COVID diagnosis.
Sharp follows the public health recommendation of the four-tier approach for prioritizing COVID-19 testing. The Infectious Diseases Society of America (IDSA) has created the following guidelines and recommends taking into account the number of confirmed cases in the community. This is updated as testing becomes more widespread and new information is available.
The first tier includes:
- Critically ill patients in ICU-level care who have unexplained symptoms of viral pneumonia or respiratory failure regardless of travel history or close contact with patients with suspected or confirmed COVID-19 infection.
- Individuals, including health care workers, with a fever or respiratory symptoms and have had close contact with a patient who has a laboratory-confirmed case of COVID-19 within 14 days of symptom onset, including all patients in long-term care facilities or recent travel to high transmission areas.
- Patients with fever or respiratory symptoms that are immunosuppressed or elderly with underlying chronic conditions or health care professionals critical to the pandemic response.
Tier two includes hospitalized patients who are not in the ICU and long-term residents with an unexplained fever and symptoms of a lower respiratory tract infection. The IDSA says, “As testing becomes more widely available, routine testing of hospitalized patients may be important for infection prevention and management at discharge.”
Tier three includes outpatients who are eligible for influenza testing, such as those with conditions like diabetes, chronic obstructive pulmonary disease, and congestive heart failure. Also included in this group are patients older than 50 years with immunocompromised issues. The IDSA notes that “testing of pregnant women and symptomatic children with risk factors is encouraged.”
Tier four includes individuals in communities being monitored by public health and infectious disease authorities.
Flu Season
Sharp employees will begin getting flu vaccines this week and it is recommended for the community to get the vaccine as a safeguard against potential flu viruses this flu season. Sharp Coronado Hospital’s annual Community Flu Clinic will be held on October 5 and October 9 from 11 am to 4 pm. The clinic will offer free flu shots and will be stationed outside of the Sandermann Auditorium entrance on Soledad Place. Please note that high dose flu vaccines will not be available at these clinics. The Sharp Coronado Community Pharmacy will be offering flu shots Monday through Friday from 10 am to 4 pm. High dose flu vaccines are available at the Community Pharmacy for those wishing this vaccine and are 65 years of age or older.
The Centers for Disease Control (CDC) outlines types of flu shots for people 65 years and older:
People 65 years and older should get a flu shot, not the nasal spray vaccine. They can get any flu shot approved for use in their age group with no preference for any one over another. There are regular flu shots that are approved for use in people 65 years and older and there are also two vaccines designed specifically for this group:
- High Dose Flu Vaccine
The high dose vaccine (brand name Fluzone High-Dose) contains four times the amount of antigen (the inactivated virus that promotes a protective immune response) as a regular flu shot. It is associated with a stronger immune response following vaccination (higher antibody production). Results from a clinical trial of more than 30,000 participants showed that adults 65 years and older who received the high dose vaccine had 24% fewer influenza illnesses as compared to those who received the standard dose flu vaccine. The high dose vaccine has been approved for use in the United States since 2009.
- Adjuvanted Flu Vaccine
The adjuvanted flu vaccine (brand name Fluad) is made with MF59 adjuvant, an additive that can create a stronger immune response to vaccination. In a recent review of multiple vaccine trials, older adults who received a MF59-adjuvated vaccine had a significantly higher immune response than those who received a standard flu vaccine, The adjuvanted vaccine was available for the first time in the United States during the 2016-2017 flu season.
“The COVID 19 pandemic has brought unique challenges and our teams have risen to meet them in creative ways,” comments Stone. The Auxiliary team was challenged when they were required to close the hospital Gift Shop and thrift store, Second Best Shop. In response, they have created new and innovative ways to support the Auxiliary. Space has been dedicated in the Coronado Community Pharmacy featuring Gift Shop items for purchase. In addition, a phone-in option is now available to purchase items from an online Gift Shop where people can view items and click on the link or just call (619) 522-3611 to make a purchase and have a gift delivered directly to a patient’s room. In addition, Second Best Shop is pleased to announce the grand opening of its virtual thrift shop. The online Second Best Shop features a variety of offerings, including clothes and household items, for purchase directly on the website and then the team will coordinate with customers for a touchless pick up.
Sharp Coronado Hospital Gift Shop





